Our Patent Families
We proudly hold 29 registered patents, each marking a significant leap in technological innovation. These patents span diverse areas, showcasing our commitment to advancing imaging techniques, AI algorithms, hardware solutions, and software frameworks. They reflect our drive for excellence and secure our competitive edge while propelling the industry forward. With this extensive patent portfolio, we lead in driving transformative change and setting new standards in innovation.



Nanox.SOURCE

Nanox.SOURCE
A proprietary silicon-based, low voltage, nano-scale cold cathode generating the electron stream needed for X-ray via field-emission technology
Read More about Nanox.SOURCEFDA
510(k) cleared imaging systems using the Nanox.SOURCE
10
Patent families, registrated in various countries
9
Years developing the source for the medical imaging industry
FED TECHNOLOGY
Over a decade of substantial resources invested in development of FED technology by Sony
Nanox.TUBE
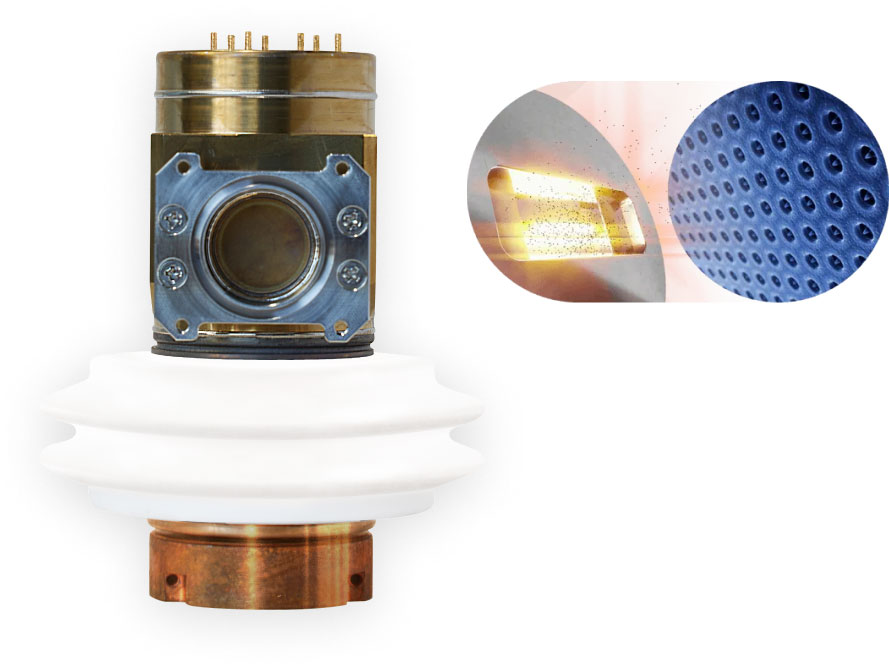
Nanox.TUBE
From a single metal filament heated to 2,000° Celsius, requiring special cooling and rotation mechanics, to a silicon chip featuring a field of 100 million nano-cones emitting digitally controlled electron streams under low voltage.
Read More about Nanox.TUBEFuture Benefits of Cold Cathode X-ray Tubes
Size Improvements
Most beneficial in mobile imaging units that run off chargeable batteries.
No filament heating is required to “boil off” electrons from the cathode.
Energy Efficiency
Cold Cathode tubes are much smaller and lighter then conventional X-ray tubes.
Drastically reduces the size and complexity of imaging systems, and consequently, their cost.
Multi-source Imaging
Allows simultaneous connection and switching of multiple X-ray tubes.
Only two voltages control X-ray output: the Cathode-Anode voltage and the Cathode Cone-Gate voltage.
Nanox.CLOUD

Nanox.CLOUD
A proprietary software platform designed to streamline the radiology diagnostics services, image processing and Nanox.ARC fleet management.
Read More about Nanox.CLOUDNanox.CLOUD Enables
Cloud based image reconstruction
No need for costly hardware

Continuous updates
Centralized protocol management
Provide and maintain scan protocols to the Nanox.ARC devices.

Remote installation capability
24/7 remote support
HIPAA & GDPR compliance
Nanox.AI

Nanox.AI
Nanox AI’s solutions are developed to target highly prevalent chronic and acute diseases affecting large populations around the world. Leveraging AI technology, Nanox AI helps clinicians extract valuable and actionable clinical insights from routine medical imaging that otherwise may go unnoticed, potentially initiating further medical assessment to establish individual preventative care pathways for patients.
Read More about Nanox.AIPioneering Technologies Driving the Future of Innovation
Artificial Intelligence
Nanox.AI develops artificial intelligence algorithms in multi-modality of medical imaging, using state of the art technology
Deep Learning
The technology uses deep learning networks that rely on the Nanox.AI medical imaging data lake
Data
Lake
The Nanox.AI data lake contains millions of anonymized HIPAA-compliant medical images and reports

Proprietary Technology
Proprietary technology uses NLP to process medical reports on a large scale, enabling smarter data curation
Customer Needs
Nanox.AI develops technology aligned with customer needs and requirements
Seamless Workflow
Providing a seamless workflow in the healthcare system with wide and flexible customization and integration capabilities
Cutting-Edge Medical AI Technology
Over a decade of Al imaging experience


Collaborations
Together, We Redefine Possibilities










 Client portal
Client portal